How to Draw Lewis Structures 1. Place the remaining atoms on the top bottom left and right sides.

How To Draw Lewis Structures For A Molecule With One Central Atom No Octet Rule Exceptions Chemistry Study Com
Draw the Lewis structure for BBr.
. -Hydrogen cannot be central. The central atom in a lewis structure is always what as long as it is not hydrogen. Place a pair of electrons between each atom to form a bond.
Hydrogen and Halogens are never central atoms. Choose a central atom well start with small molecule examples for which there is only one central atom and the other atoms - the peripheral atoms -. Use two valence electrons to form each bond in the skeleton structure.
The nitrogen N atom is situated at a central position and the hydrogen H atoms are at the outside position in the lewis diagram. Boron is the central atom and select the correct model representing its electron pair geometry and molecular geometry. A Lewis diagram is a representation of the valence shell electrons in a molecule where each atom participating in the bond tries to acquire the octet structure.
In lewis structure of NH 3 three bond pairs and one lone pair are present. When drawing a lewis structure of polyatomics what do you do that differentiates it from other lewis structures because it. Which atom will never be in the middle of a Lewis dot structure.
Atom with the greatest mass c. Steps for drawing the Lewis dot structure. It is also the atom having low electronegativity.
The least-electronegative atom is central except for hydrogen which is never central. Draw a Skeletal. Electron Pair Geometries Linear Trigonal planar Tetrahedral Tetrahedral Tetrahedral Molecular Geometries Linear Trigonal planar Tetrahedral Bent Trigonal pyramidal.
Rules for building Lewis dot structures. That list is. Placing the Elements in the Drawing.
If all of the atoms usually form the same number of bonds the least electronegative atom is usually the central atom. Calculate the total number of valence electrons in the molecule by adding the valence electrons from each atom. In drawing Lewis structures for relatively small molecules and polyatomic ions the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms.
1 When drawing the Lewis structure of compounds we normally start by knowing the total valence electrons present in the molecule. This way you would expect chlorine to be the central atom. The drawing of the NH 3 Lewis structure is very easy and simple.
Place least electronegative element in center and draw single bonds from the central atom to other atoms. Determine which atom will be the. A If there are more than one of the least electronegative atom your skeletal structure should have those two attached to one another EXCEPTION.
Usually the central atom will be the one that has the most unpaired valence electrons. Ethers O is the central atom between two C Alcohols O is the central atom between C and H Aldehydes ketones and amides O is attached terminally to one C in an amide the C is also bonded to an N Esters and carboxylic acids two O are attached to one C one O is terminal and the other is attached to another atom. When drawing lewis structures which atom is usually the central atom.
Put a box around the lewis structure with a charge. Determine the total number of valence electrons to be depicted in the Lewis diagram. In drawing the Lewis structure the central atom is generally the.
What this means is that we decide how the atoms are to be bonded. The order of electronegativity for the nonmetals is FONClBrISCH. Write the Lewis structure for CH2O where carbon is the central atom.
In our case the O atom will be the central atom and the total valence electrons are 8e. Determine how many electrons must be added to central element. See answer 1 Best Answer.
Best Answer Copy The central atom in a Lewis structure is usually the least electronegative atom. Then we determine the central atom which is likely to be the atom that forms most bonds. Determine the total number of electrons available for bonding.
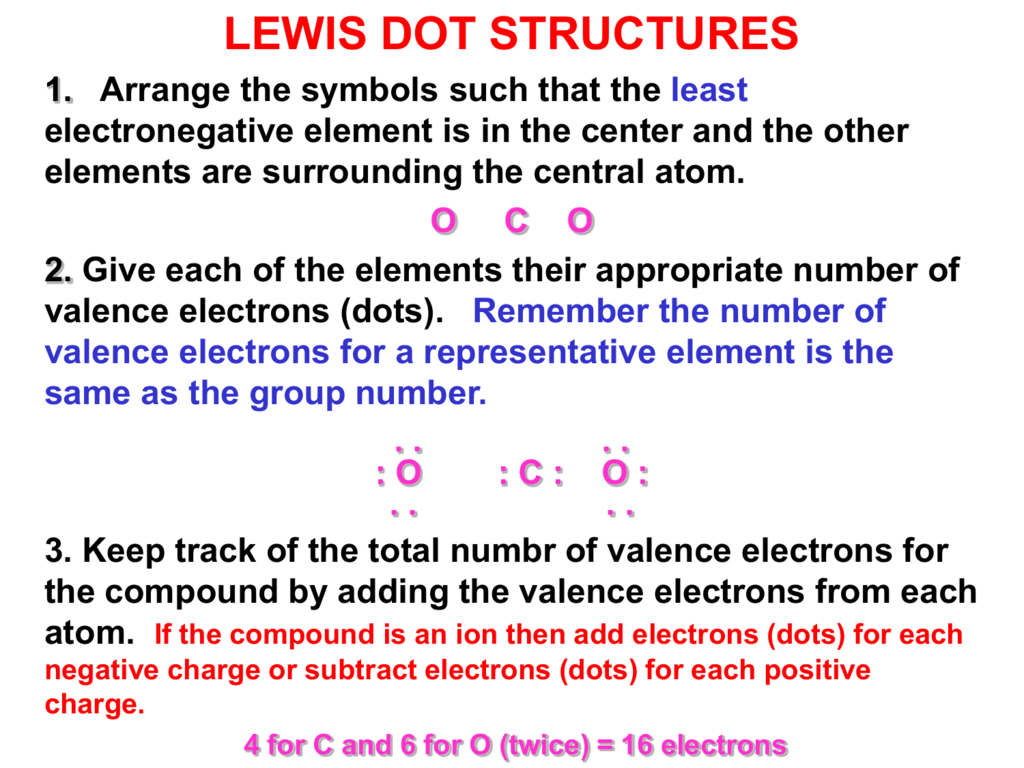
CO 2 Total 16. Lewis dot structures can be drawn to show the valence electrons that surround an atom itself. The central atom of a molecule is usually the least electronegative atom or the atom with the highest valence.
Draw basic Lewis structure for each atom. Determine the central atom. The Lewis dot structure of Boron trichlortde enhances the idea about sharing electrons.
Each bond uses two valence electrons. Next place one pair of electrons between the atoms and join them to indicate the formation of a chemical bond. These elements are also in priority order.
The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. In drawing a Lewis structure the central atom is the a. How to Draw a Lewis Dot Structure Step 1.
Atom with the highest atomic number d. This list will cover your needs on about 95 of questions you encounter for e lectron d ot s tructures Lewis Structures. Hereof how do you find the central atom when drawing a Lewis structure.
Lets see how to do it. If an atom occurs only once this atom is. Draw a skeletal structure.
So if you have covalent compound with C and P then C is the central atom because it comes first on the list. C Si N P S O. As shown in the following video the central atom in Lewis Dot Structures is always the least electronegative atom of all the elements that make up the atom.
Hydrogen is never the central atom. Can hydrogen be a central atom for. The central atom is also usually the least electronegative.
To find electronegativity either rely on periodic table trends or consult a table that lists electronegativity values. Boron is the central atom which needs three electrons to have total six electrons in its last electron shell it does not necessarily needs to fulfil its octet. I usually prefer to teach my students this way at first to just get them rolling.
Boron has three valance electrons and three chlorine atoms have 37 21 valance electrons. Metals will almost always be the central atom if they are present. Complete the octet of ench atom.
When constructing a Lewis diagram keep in mind the octet rule which refers to the tendency of atoms to gain lose. The central atom in a Lewis structure is usually the least electronegative atom. Atom with the fewest electrons b.
Rules for Drawing Lewis Structure. Steps of drawing lewis structure of Cl 2 O.
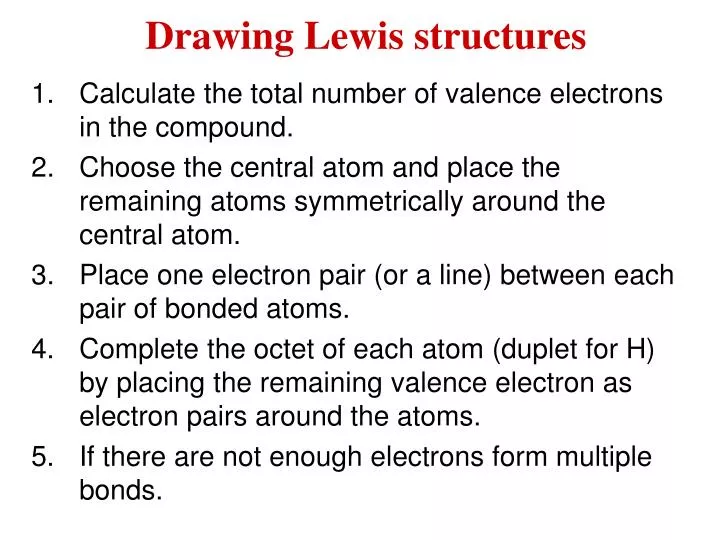
Lewis Structures Multiple Central Atoms Science Chemistry Chemical Bonds Showme

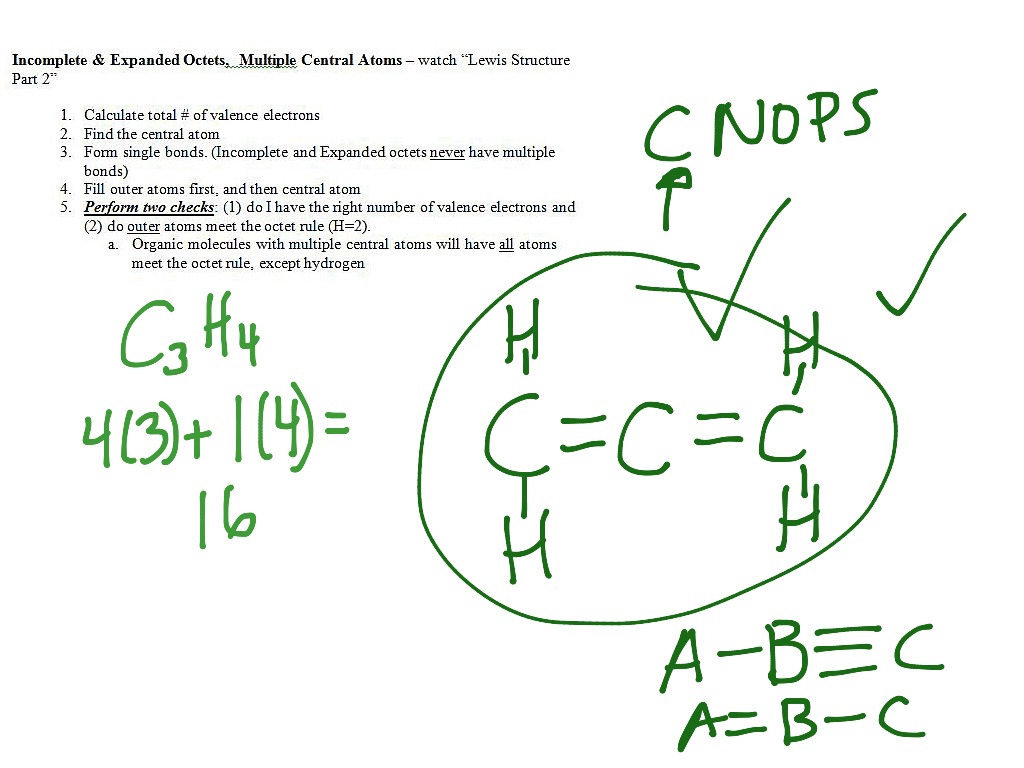
Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421
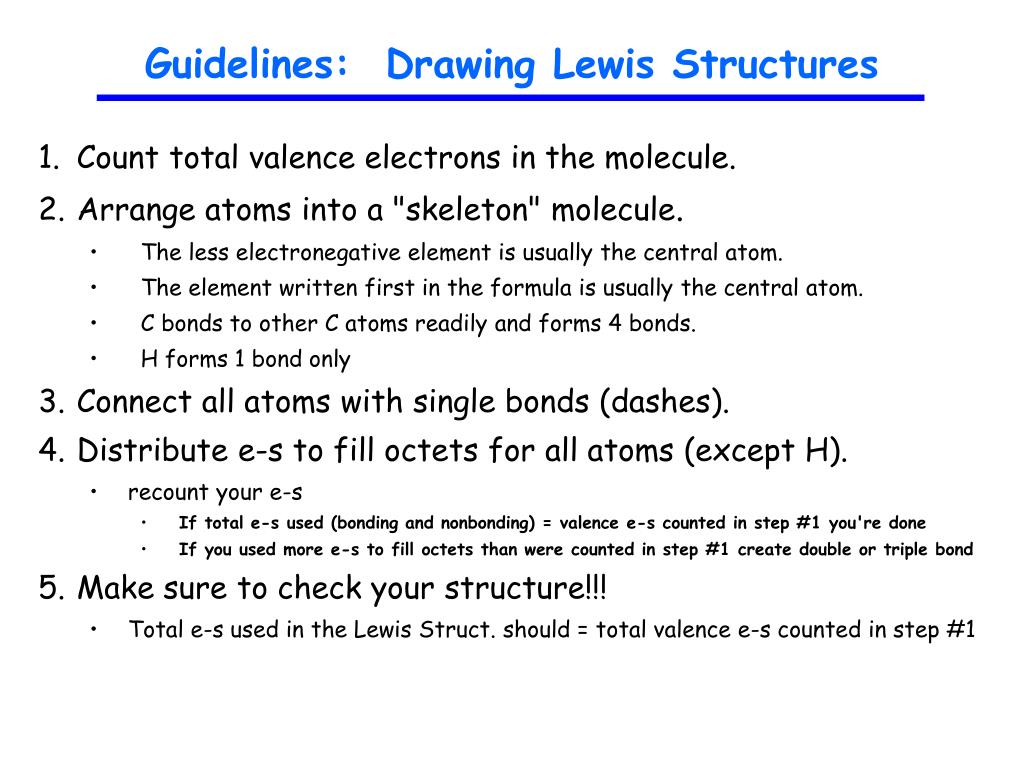
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949
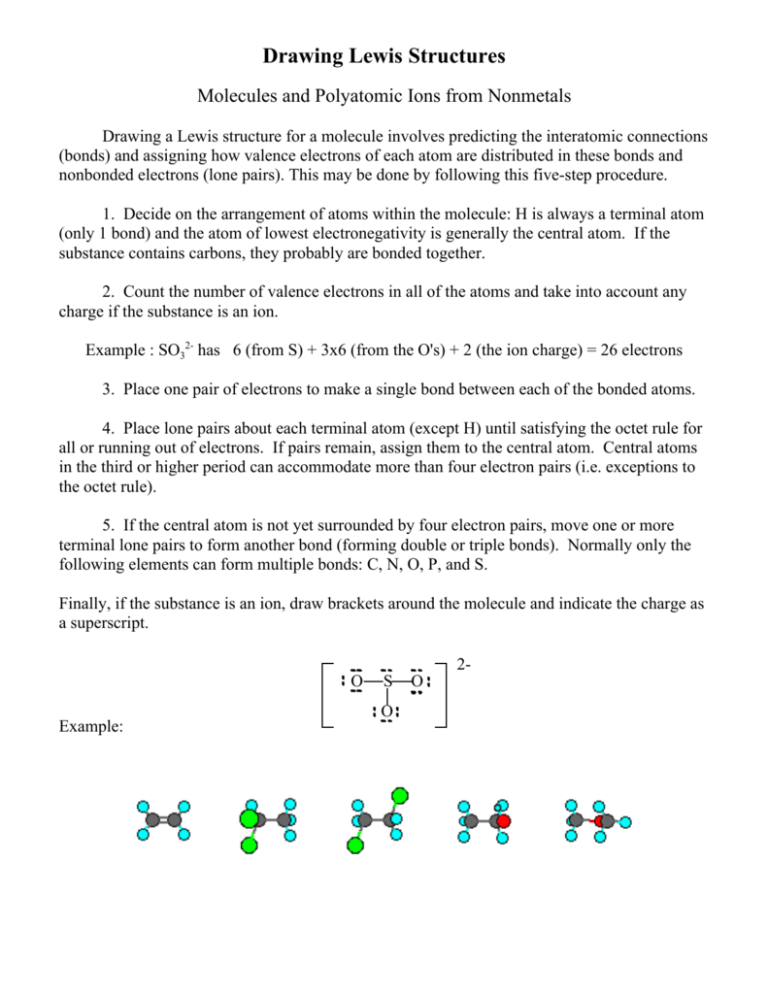
Rules For Drawing Lewis Structures

Writing Lewis Structures Ppt Video Online Download

Step 1 Identify The Central Atom And Draw It S Lewis Structure This Is The Element With The Lowest Number Of Atoms In The Formula Draw A Lewis Dot Diagram Ppt Download

Rules For Lewis Structures Pre Ap Adapted From Brown And Lemay Ppt Download
0 comments
Post a Comment